Current ITP Portfolio
Explore our current portfolio of Interdisciplinary Translational Projects (ITPs) driving innovation in dental, oral, and craniofacial space focused on regenerative medicine and tissue engineering. Each project is led by a dedicated project team collaborating with TRC experts, combining the expertise of clinicians, scientists, engineers, and regulatory specialists to address unmet clinical needs. Centered on preclinical product development and translational impact, our projects aim to advance promising technologies toward clinical adoption and improved patient care.
About this table
Project maturity is defined by a specific phase, using the Translational Development Status Questionnaire developed and published by the TRC. This tool tracks overall progress through five key stages, providing a high-level overview of translational maturity based on essential project deliverables. For further details, please see our publication in the Journal of Dental Research.
- Research & Planning
- Preclinical Development: Feasibility
- Preclinical Development: Verification
- Preclinical Development: GLP Validation
- Clinical Development: 510(k)/IDE/IND Preparation
For comprehensive descriptions of each phase and its deliverables, download the full document below.
Projects
| Overview | Project | Tissue Adressed | Technology Type | Regulatory Path | Research & Planning | Preclinical Development: Feasability | Preclinical Development: Verification | Preclinical Development: Validation | Clinical Development: Preparation |
|---|---|---|---|---|---|---|---|---|---|
| Ostiio: A Smart, Fully-Implantable CMF Distractor | Ostiio: A Smart, Fully-Implantable CMF Distractor | Bone | Device | 510(k) | Phases 2-3 | ||||
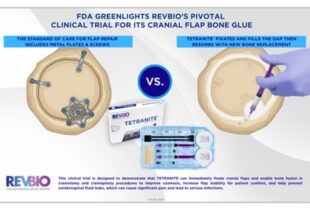
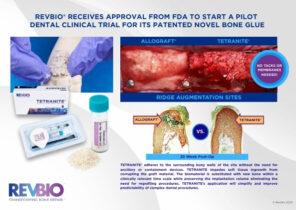
| TETRANITE®: Optimization of a Novel Organic-Mineral Bone Adhesive for Dental Bone Grafting | TETRANITE®: Optimization of a Novel Organic-Mineral Bone Adhesive for Dental Bone Grafting | Bone | Combination (device/ drug) | IDE | Phases 2-5 | ||||
| Non-Viral Aquaporin-1 Gene Therapy to Restore Salivary Flow | Non-Viral Aquaporin-1 Gene Therapy to Restore Salivary Flow | Salivary Gland | Biologic | IND | Phases 1-4 | ||||
| Suture-less Trigeminal Nerve Repair Device | Suture-less Trigeminal Nerve Repair Device | Soft Tissue | Device | 510(k) | Phase 2 | ||||
| Amend Tissue Tape™ for Oral Wound Care | Amend Tissue Tape™ for Oral Wound Care | Soft Tissue | Device | 510(k) | Phases 3-5 | ||||
| Commercialization of Vital-Dent | Commercialization of Vital-Dent | Teeth/ Dental Pulp | Device | 510(k) | Phases 1-3 | ||||
| Targeted Remineralization Treatment Using Mineral Loaded Starch Nanoparticles | Targeted Remineralization Treatment Using Mineral Loaded Starch Nanoparticles | Teeth/ Dental Pulp | Device | Class 1, 510(k), Monograph, De Novo | Phases 2-5 | ||||
| RegendoGEL: A Bioinspired Hydrogel System for Endodontic Therapy | RegendoGEL: A Bioinspired Hydrogel System for Endodontic Therapy | Teeth/ Dental Pulp | Device | IDE | Phases 1-3 | ||||