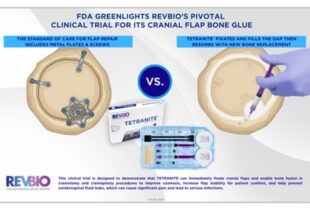
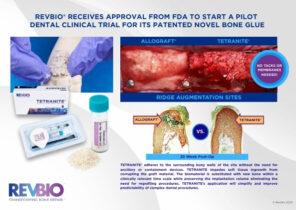
Story
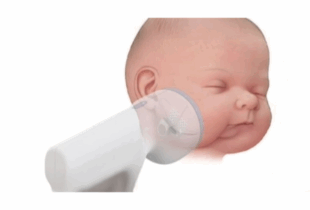



Ostiio’s Odyssey: Trailblazing a Transformation in Craniofacial Distraction Technology
A collaboration between Dr. Jesse Taylor, Chief of Plastic Surgery at the Children’s Hospital of Philadelphia (CHOP) and Dr. Ari Wes, Plastic Surgery Resident at the Hospital of the University of Pennsylvania (Penn) began in 2012, when they set out to study multiple aspects of craniofacial disease, treatment paradigms, and surgical outcomes.
Distraction osteogenesis (DO) in the craniomaxillofacial skeleton soon emerged. Although its benefits over traditional treatment modalities, such as reconstructive surgery, were evident, additional research underscored the limitations of DO in its current form. Currently available distractors have external components that protrude through the skin for manual engagement of the distractor – using a screwdriver – typically twice a day for multiple weeks. As a result, Dr. Wes pursued a Master of Science in Translational Research, where his thesis, Novel Considerations in Craniomaxillofacial Distraction Osteogenesis, envisioned a fully subcutaneous, automated DO system for the CMF skeleton and formed the foundation of what would later become Ostiio.
Ostiio, LLC was founded in 2017, when it was spun out of Penn to improve outcomes and experience for pediatric patients with craniofacial defects resulting from impaired growth of their skull or mandible. Through interviews with parent caregivers and surgeons, the team came to understand the critical challenges with DO – predisposition to morbidity such as soft-tissue infection due to the external component, unintentional patient/parent noncompliance, patient discomfort/increased analgesic use throughout the distraction period, as well as the resulting scarring. The company was built around an idea to help the smallest of patients and their caretakers and doctors, to change the world of craniofacial distraction.
With a fundamental prototype and unmet needs defined, the project was selected for funding by the ITP program in 2019. Learnings from the previous studies with the caregivers and surgeons helped the team define clinical performance criteria that would later form the basis of the most critical design inputs: the envisioned product would address the primary user objections to distraction – semi-buried implant, high caregiver burden, and limited surgeon control. However, at this earlier stage, it was not clear if the concept could be translated into a working prototype that would meet performance requirements and fit within the craniomaxillofacial anatomy. Miniaturization cannot come at the expense of meeting the distraction force performance specifications, so this design need has been a major challenge to overcome.
Through the ITP program, the team built and tested its first proof-of-principle implant and handheld driver (HHD) prototypes, achieving the needed distraction force, validating the proof of principle. At the same time, the team raised additional funding through pre-seed round of financing to further support its product development. With the technical advancement of the prototype, the team held its first pre-submission meeting with the FDA, where they received guidance for their roadmap towards a 510(k) submission, which will likely not require a human clinical trial.
In 2020, Ostiio brought on a CEO, Jessica DesNoyer, a seasoned professional with expertise in leading medical device development. With Jessica’s leadership and the support from the MPWRM Resource Center (RC), Ostiio formalized its product development process with a quality management system and transitioned the product development to a professional engineering firm. The team collaborated with the RC Regulatory Core to better understand the regulatory requirements for the 510(k), and a subsequent pre-submission meeting with the FDA helped the team solidify their regulatory strategy to pursue multiple indications.
To integrate customer input into their product design, the Ostiio team conducted a formative human factors study and an in-depth market assessment in collaboration with the RC’s Market Assessment Core, leveraging the RC network of clinicians. This study expanded their clinician and geographical reach beyond the earlier studies that were conducted primarily at Children’s Hospital of Philadelphia (CHOP). Further, the findings re-emphasized the known challenges with DO, and helped the Ostiio team better refine their market fit and value. With this support from the RC, the Ostiio team is poised to integrate the learnings from multiple aspects of the project, including regulatory, market assessment, and technical development, to develop a device that not only meets user needs, but also streamlines their pathway towards a 510(k) submission.
The ITP program has been an invaluable contributor to the success of our company. From the support provided by the Resource Center to the continued funding as we bridge the technology “valley of death”, the ITP program has been a true partner in facilitating Ostiio’s growth from early stage development into an investable MedTech company.
Under Jessica’s helm, the company has won multiple SBIR grants from both NSF and NIH to support different aspects of the fully-implantable distractor device. The team has also been named a MassChallenge 2021 Early Stage Program Gold Award Winner. From a cohort of 229 startups across all industries and technologies, this was formal recognition of the meaningful impact of Ostiio’s work on a national stage.
Current plans with the ITP program will enable the team to complete a functional prototype system that achieves the performance specifications ready for preclinical testing. Upon verification of a system prototype that can complete a full distraction protocol in vivo without need for invasive manipulation, the team plans for a final prototype iteration (Gamma), which will be representative of the entire, final product and as close to the commercial product as possible. This prototype will incorporate any design changes resulting from findings of the animal study and a formative usability study and will be tested against performance requirements prior to design freeze. With these plans, Ostiio aims to submit at 510(k) in 2025, and strives to get this revolutionary device into the hands of patients, parent caregivers, and surgeons.
By addressing the primary customer and patient concerns with DO, including the semi-buried form factor of distractors, Ostiio can also drive adoption of the technique for on-label adult indications, such as obstructive sleep apnea (OSA). The team is optimistic that within five years-time, the Ostiio distraction system will have significantly penetrated the pediatric market, accelerating adoption of DO over alternative treatment modalities and markedly improving quality of care for these patients. Further, as data mount supporting the safety and efficacy of mandibular lengthening as a treatment for adult OSA, their fully subcutaneous solution will make DO a viable choice for surgeons and patients where it is not a choice today.