2022 4th MPWRM Resource Center Annual Stakeholder’s Summit
April 18 – 19, 2022 | Virtual Meeting
As a follow-on to December 2021 DOCTRC session on Critical Considerations in Preclinical Product Development, this year’s Annual Stakeholder’s Summit focused on building a compelling pitch deck. Day 1 included updates from the Resource Center and presentations by the ITP teams, and day 2 comprised of 2 keynote talks and mini-sessions on components of a compelling pitch deck, followed by a breakout discussion.
Event agenda:
April 18, 2022 (Day 1)
- ITP Progress Updates
- ITP teams: Stakeholder Listening Sessions
- Closed door session: MPWRM Resource Center business
April 19, 2022 (Day 2)
- Keynote Presentation: Fundraising for Advancing your DOC Technology Towards Commercialization and Successful Clinical Adoption
- Critical Considerations in Preclinical Product Development, II: Building a Compelling Pitch Deck
- Session 1: Regulatory Strategies
- Session 2: Market Assessment
- Session 3: User Handling Trials
- Breakout Session: Building a Compelling Pitch Deck
- Closed door session: MPWRM Resource Center business
Welcome Remarks | April 18, 2022 (Day 1)
- Lillian Shum, PhD | Director, Division of Extramural Research, NIDCR
Keynote Presentation: Attracting and Preparing for Investor Meetings & Successful Fundraising Presentations and Strategies: Point to Consider | April 19, 2022 (Day 2)
- Gene Dorff | Chief Executive Officer | Solmetex, LLC
- Marty Dymek | CEO | Navigate Surgical Technologies USA, Inc.
Considerations in Preclinical Product Development, II: Building a Compelling Pitch Deck | April 19, 2022 (Day 2)
- Session 1: Regulatory Strategies | Kay Fuller (MRDS LLC)/ ITP Panel: Emergence Dental/nanoMag, Luiz Bertassoni/ Pamela Yelick
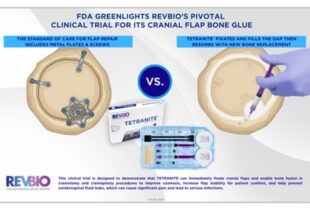
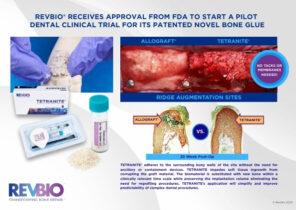
- Session 2: Market Assessment | Francine Berkey (The Avenues Company)/ ITP Panel: Will Giannobile, Juan Taboas/ Herb Ray
- Session 3: User Handling Trials | Jeanne Ambruster (The Avenues Company)/ ITP Panel: Ostiio, RevBio