RevBio’s Tetranite® : Redefining Bone Repair and Regeneration Through Groundbreaking Bioadhesive Innovation
RevBio is a science and engineering start-up focused on solving the unmet clinical need for a biomimetic bone adhesive that can be used to augment bone ridges.
 The solution, Tetranite®, is a cohesive, injectable, and self-setting material that can be applied in wet surgical environments. It does not get washed away during the application process, then hardens within minutes to create adhesive bonds to surrounding bone in the shape of a bone ridge as it cures. As part of the MPWRM Resource Center (RC), RevBio is exploring these robust characteristics of Tetranite to enhance oral bone regeneration in challenging situations where conventional particulate or block grafts have been ineffective or unpredictable, at best.
The solution, Tetranite®, is a cohesive, injectable, and self-setting material that can be applied in wet surgical environments. It does not get washed away during the application process, then hardens within minutes to create adhesive bonds to surrounding bone in the shape of a bone ridge as it cures. As part of the MPWRM Resource Center (RC), RevBio is exploring these robust characteristics of Tetranite to enhance oral bone regeneration in challenging situations where conventional particulate or block grafts have been ineffective or unpredictable, at best.
The development of Tetranite was already in progress for other indications when the project entered the ITP program in 2018 with a focus on its development for dental bone grafting applications. Through market research of their own, the RevBio team learned that 70% of all patients who receive dental implants require some form of bone grafting prior to implant placement, yet most dentists remain dissatisfied with existing bone graft products – especially in non-space making defects – due to their challenging handling properties. As most products lack structural integrity, they typically require the use of fixation or containment devices to stabilize the graft and membranes to prevent ingrowth of fibrous tissue that impedes bone regeneration and remodeling. The application of Tetranite with its distinctive adhesive properties is expected to obviate the need for these devices, making the procedure less technique sensitive with more predictable outcomes.
With the RC’s Market Assessment Core, the team conducted rounds of interviews with clinical and industry experts in the dental bone grafting space. From these discussions, the RevBio team gained a deeper understanding of the challenges in dental bone grafting and the associated market dynamics, including the limitations of competitive products and their pricing. With the learnings, the team continued to hone their target indication, where the unique properties of Tetranite can best be leveraged and its value optimized. The Market Assessment Core also provided the team with market data related to specific indications as well as bone graft types and projected growth opportunities in the market.
 Initial studies conducted in the ITP program allowed the formulation to be fine-tuned, material characterized, and proof-of-concept efficacy studies to be completed. With this formulation, the project proceeded with a GLP preclinical study using a model discussed with the FDA in a pre-submission meeting. Due to the unique preclinical model, the GLP facility and vendor was selected in collaboration with the RC’s Regulatory and Quality Assurance Cores and the selected facility was pre-qualified by the Quality Assurance leads of RevBio and the RC’s Quality Assurance Core. As this study got underway, issues with the preclinical model emerged. Upon an in-depth review of the complications with the RC’s Regulatory and Market Assessment Cores and clinical experts, the study model was thoughtfully re-designed. With support from the RC, the team successfully obtained concurrence from the FDA that the revised model would be appropriate for the GLP preclinical study.
Initial studies conducted in the ITP program allowed the formulation to be fine-tuned, material characterized, and proof-of-concept efficacy studies to be completed. With this formulation, the project proceeded with a GLP preclinical study using a model discussed with the FDA in a pre-submission meeting. Due to the unique preclinical model, the GLP facility and vendor was selected in collaboration with the RC’s Regulatory and Quality Assurance Cores and the selected facility was pre-qualified by the Quality Assurance leads of RevBio and the RC’s Quality Assurance Core. As this study got underway, issues with the preclinical model emerged. Upon an in-depth review of the complications with the RC’s Regulatory and Market Assessment Cores and clinical experts, the study model was thoughtfully re-designed. With support from the RC, the team successfully obtained concurrence from the FDA that the revised model would be appropriate for the GLP preclinical study.
The RevBio team also conducted material characterization and biocompatibility testing, and developed a unique sterile packaging for the product. To refine the prototype, the RC helped organize several user validation and human factor engineering tests, including in-person user handling trials. In collaboration with the RC Market Assessment Core, the team developed plans and recruited >10 clinicians to participate in its in-person user handling trials, leveraging co-timing with professional meetings where many of these key opinion leaders (KOL) clinicians were in attendance. The practitioners were brought together, and provided with the RevBio prototype in its packaging, where they had first-hand experience using it in a simulated clinical environment. The team received valuable feedback from the clinician participants, which resulted in changes to the kit design and reduction of the packaging footprint, as well as many good ideas regarding education and training that will drive broader clinical adoption.
Overall, the Resource Center and its internal resources have played an important role in advancing the development of this high-potential product.

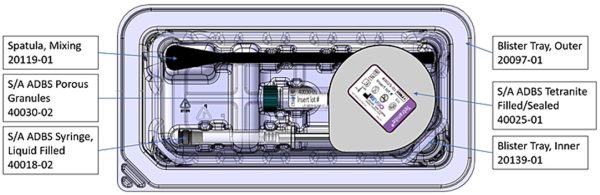 RevBio continues to push boundaries and explore multiple indications for Tetranite. The product is in clinical stages for implant stabilization, cranial flap fixation, and veterinary use. For its bone indications, the team also conducted an in vivo experiment to study Tetranite’s ability to regenerate bone in microgravity conditions — on the International Space Station (ISS). In fact, this is Tetranite’s second trip to space, following a previous in vitro experiment. Sponsored by the ISS National Laboratory, Tetranite was launched on SpaceX’s 26th Commercial Resupply Services (SpaceX CRS-26) mission, where the microgravity in space is used to simulate conditions where bone growth and regeneration is compromised.
RevBio continues to push boundaries and explore multiple indications for Tetranite. The product is in clinical stages for implant stabilization, cranial flap fixation, and veterinary use. For its bone indications, the team also conducted an in vivo experiment to study Tetranite’s ability to regenerate bone in microgravity conditions — on the International Space Station (ISS). In fact, this is Tetranite’s second trip to space, following a previous in vitro experiment. Sponsored by the ISS National Laboratory, Tetranite was launched on SpaceX’s 26th Commercial Resupply Services (SpaceX CRS-26) mission, where the microgravity in space is used to simulate conditions where bone growth and regeneration is compromised.
In addition to the support from the MPWRM Resource Center and the National Institute of Dental and Craniofacial Research, RevBio has garnered significant federal grant funding from several other institutes of NIH, including National Institute on Aging and the National Institute of Neurological Disorders and Stroke. They have also been successful during several rounds of fundraising. The team is results-driven and has taken full advantage of the RC expertise in designing pre-clinical studies, handling sessions, regulatory strategies, and quality oversight, moving quickly towards their first-in-human studies. For the dental bone grafting indication, the team is striving towards an IDE submission in late 2024.
For any questions/interests in the projects, please contact us at
translationalrc@umich.edu
